Table of Contents
Introduction:
- COVID-19 vaccines of Pfizer and Moderna companies are the 1st and 2nd vaccines in the world approved for human use to prevent COVID-19 infection/disease.
- In December 2020, the Food and Drug Administration (FDA) of United States granted Emergency Use Authorization (EUA) for two COVID-19 vaccines.
- An EUA means the FDA has determined the vaccines are safe and effective enough to recommend for preventing COVID-19. No COVID-19 vaccine has received full FDA approval as past vaccines have, which takes more time.
- These vaccines are safe and effective for preventing COVID-19 infection/disease.
There are lot of similarities and differences between the two vaccines. Let’s have a look at it.
Similarities between COVID-19 Vaccine of Pfizer and Moderna:
1. Both vaccines are made using messenger RNA or mRNA.
Note: mRNA is a technology that delivers a bit of genetic code to cells — in effect, a recipe to make the surface protein (known as spike) on the SARS-2 virus. The proteins made with mRNA teaches the immune system to recognize spike protein (the spike that we see (in online images) in the body of Corona virus) as a foreign material.
2. During the clinical trials of both vaccines, none of the people needed hospitalization.
3. The most common side effects of both vaccines are:
-
- Headache
- Fever
- Chills
- Muscle pain
- Joint pain
- Mild pain in the injection site
- Tiredness
- Nausea or vomiting
Note: These side effects are just a sign of immune system kicking to gear mode against corona virus. It does not mean that the vaccine is unsafe.
4. Both vaccines require specific cold chain. However, their cold chain temperature varies.
5. None of the vaccines are tested in pregnant and breastfeeding women & children, individuals taking anti-clotting medications or immune-suppressing medicines.
6. For pregnant and lactating individuals, although there is no human data, researchers claim that interim data from animal studies shows no issues for safety. However, it pregnant and lactating women should consult their medical professional before taking the vaccine.
7. Status of both the vaccines are: Emergency use in the U.S.; authorized for use in the European Union.
8. If necessary, both the vaccines can be given up to 6 weeks (42 days) after the first dose.
9. In case of both vaccines, individuals will be fully protected only after 2 weeks after the completion of the second dose of vaccination.
Remember: Although both vaccines are made using mRNA, the mRNA molecules in both vaccines are slightly different in structure and makeup. mRNA in both vaccines have different so-called lipid delivery systems, meaning the sort of fatty droplet in which the messenger RNA is located. That’s why they have different storage and handling characteristics.
13 Major Differences between COVID Vaccine of Pfizer and Moderna:
Basis of Difference |
Pfizer |
Moderna |
| Partnership | Pfizer was developed in partnership with German-manufacturer BioNTech. | Moderna was developed with assistance from the National Institute of Allergy and Infectious Diseases. |
| Age groups | Pfizer vaccine can be given/administered to anyone age 12 and older. | Moderna vaccine can be given/administered to people aged 18 and older. However, the company is now testing its vaccine for 12- to 17-year-olds as well. |
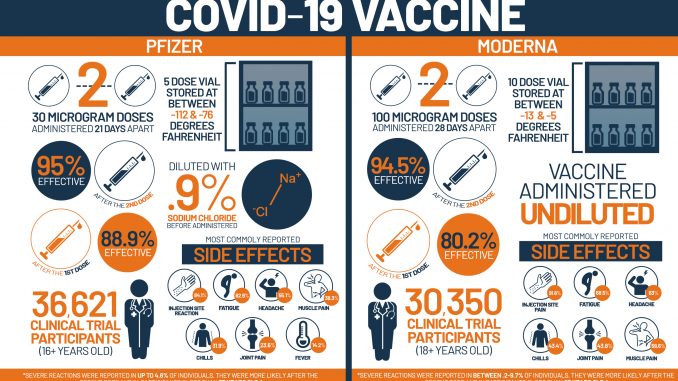
| Efficacy | Pfizer vaccine has efficacy of 95% at preventing symptomatic COVID 19 infection at least 7 days after two doses. | Moderna vaccine is 94.1% effective at preventing symptomatic COVID 19 infection at least 14 days after the second dose. |
| Hospitalization and deaths | Pfizer vaccine is 100% effective against hospitalizations and deaths from COVID-19 infection/disease. | Moderna vaccine is 89% effective against hospitalizations and 100% effective against deaths from COVID-19 infection/disease |
| Differences in efficacy among different people | Efficacy rates did not vary based on demographic factors like age, race, or ethnicity. | Moderna vaccine have slightly lower efficacy (about 86%) in people who are 65 and older. |
| Cold storage | It requires ultra cold storage. Pfizer vaccine requires -94 degree F (-75º C) for shipping and storage.
|
It requires routine cold storage. Moderna vaccine must be shipped at -4 degree Fahrenheit (-20º C), which is within the temperature of a regular refrigerator freezer. |
| Dosage | Each dose of Pfizer vaccine contains 30 micrograms of vaccine. | Each dose of Moderna vaccine contains 100 micrograms of vaccine. |
| Time gap between the dose | The time interval between two doses of Pfizer vaccination, should be at least 21 days (3 weeks). | The time interval between two doses of Moderna vaccination should be at least 28 days (4 weeks). |
| Life span once opened | After thawing, a vial of the Pfizer vaccine lasts for 5 days at standard fridge temperature. | Once opened, Moderna’s vaccine is stable for:
|
| Dilution for use | Pfizer vaccine must be diluted with saline before it is injected. | Moderna vaccine is ready to administer. |
| Dose in 1 vial | There are five doses in a vial. After dilution, one vial contains six doses of 0.3 ml. | There are 10 doses in a vial. |
| Minimum purchase order | An order of the vaccine includes 975 doses. | An order includes 100 doses. |
| Safety for use during pregnancy | Pfizer recently began a Phase 2/3 trial to test the safety and efficacy of its vaccine during pregnancy. | Moderna has completed animal studies to check the harm on pregnancy or developing fetus. The company said it saw no such signals. |
References and For More Information:
https://www.statnews.com/2020/12/18/fda-eua-moderna-vaccine-covid19/
https://share.upmc.com/2021/01/vaccines-moderna-pfizer/
https://www.yalemedicine.org/news/covid-19-vaccine-comparison
https://www.pfizer.com/news/hot-topics/covid_19_vaccine_u_s_distribution_fact_sheet
https://www.youtube.com/watch?v=UtcrfMVAmzQ&ab_channel=ABC10
https://www.youtube.com/watch?v=OtQdRNY22UM&ab_channel=CNBCTelevision
https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines.html
https://www.healthline.com/health/why-two-doses-of-covid-vaccine
https://www3.nhk.or.jp/nhkworld/en/news/backstories/1657/
https://www.goodrx.com/blog/comparing-covid-19-vaccines/
https://edition.cnn.com/2020/12/17/health/moderna-vaccine-what-we-know/index.html
https://www.astho.org/COVID-19/Vaccine-Comparison/
https://www.prevention.com/health/a35118263/astrazeneca-vs-pfizer-vs-moderna-covid-19-vaccine/
https://www.imc-healthcare.com/pfizer-vs-moderna-covid-19-vaccines/